
SL Paper 2
Boron is most often encountered as a component in borosilicate glass (heat resistant glass).
The naturally occurring element contains two stable isotopes, \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) and \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).
State the number of protons, neutrons and electrons in an atom of \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).

The relative atomic mass of boron is 10.8, to three significant figures. Calculate the percentage of \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) in the naturally occurring element.
Isotopes of boron containing 7 and 8 neutrons also exist. Suggest why releasing isotopes containing more neutrons than the stable isotope into the environment can be dangerous.
(i) State the formula of the compound that boron forms with fluorine.
(ii) Explain why this compound acts as a Lewis acid.
Markscheme
 ;
;
\(10x + 11(100 - x) = 10.8 \times 100\);
\((x = )20\% \);
Award [2] for correct final answer.
Do not allow ECF.
radioactive/radioisotope(s)/give out radiation;
Accept answers that outline the effects of radioactive pollution of the environment.
Do not accept “unstable”.
(i) \({\text{B}}{{\text{F}}_3}\);
(ii) incomplete valence shell / electron deficient / OWTTE;
capable of accepting an electron pair;
Examiners report
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a base according to both the Brønsted–Lowry and the Lewis theories of acids and bases.
The equation for the reaction between sodium hydroxide, NaOH, and nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}\), is shown below.
\[\begin{array}{*{20}{l}} {{\text{NaOH(aq)}} + {\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaN}}{{\text{O}}_3}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}}&{{\text{ }}\Delta H = - 57.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Distinguish between the terms strong base and weak base, and state one example of each.
State the equation for the reaction of ammonia with water.
Explain why ammonia can act as a Brønsted–Lowry base.
Explain why ammonia can also act as a Lewis base.
(i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is added to excess solid sodium carbonate, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}{\text{(s)}}\), an acid–base reaction occurs. Bubbles of gas are produced and the solid sodium carbonate decreases in mass. State one difference which would be observed if nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), was used instead of ammonium chloride.
(ii) Deduce the Lewis structures of the ammonium ion, \({\text{NH}}_4^ + \), and the carbonate ion, \({\text{CO}}_3^{2 - }\).
Ammonium ion\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)Carbonate ion
(iii) Predict the shapes of \({\text{NH}}_4^ + \) and \({\text{CO}}_3^{2 - }\).
\({\text{NH}}_4^ + \):
\({\text{CO}}_3^{2 - }\):
(i) Sketch and label an enthalpy level diagram for this reaction.
(ii) Deduce whether the reactants or the products are more energetically stable, stating your reasoning.
(iii) Calculate the change in heat energy, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution is added to excess nitric acid.
When 5.35 g ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(s)}}\), is added to \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) of water, the temperature of the water decreases from 19.30 °C to 15.80 °C. Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the dissolving of ammonium chloride in water.
Markscheme
a strong base: base/electrolyte (assumed to be almost) completely/100% dissociated/ionized (in solution/water) / OWTTE and a weak base: base/electrolyte partially dissociated/ionized (in solution/water) / OWTTE;
example of a strong base: any group I hydroxide / \({\text{Ba(OH}}{{\text{)}}_2}\);
example of a weak base: \({\text{N}}{{\text{H}}_3}\) / \({\text{C}}{{\text{H}}_3}{\text{N}}{{\text{H}}_2}\) / any reasonable answer;
\({\text{N}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{NH}}_4^ + + {\text{O}}{{\text{H}}^ - }\);
accepts a proton/\({{\text{H}}^ + }\) / OWTTE;
donates an electron pair;
(i) more vigorous reaction / more gas bubbles / OWTTE;
more heat released;
solid decreases more quickly;
(ii) 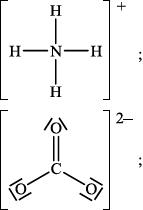
Accept any combination of lines, dots or crosses to represent electron pairs.
(iii) NH4+:
tetrahedral;
CO32–:
trigonal/triangular planar;
(i) enthalpy on y-axis;
Do not accept energy.
reactants higher than products;
\(\Delta H\) labelled;
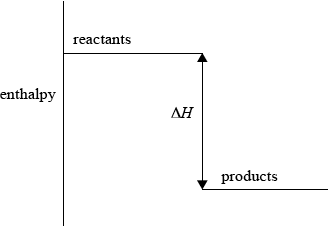
Accept appropriate formulas for reactants and products.
Arrow heads not needed.
57.6 is acceptable as an alternative to \(\Delta H\).
(ii) products are more stable as they are at a lower enthalpy level / energy has been given off by the reactants / reaction is exothermic / OWTTE;
(iii) \(n{\text{(NaOH)}} = 0.125{\text{ mol}}\);
change in heat energy \( = ( - 57.6 \times 0.125) = - 7.20{\text{ (kJ)}}\) / heat released \( = (57.6 \times 0.125) = 7.20{\text{ (kJ)}}\);
\(q = (mc\Delta T = ){\text{ }}100.0 \times 4.18 \times 3.50/1463{\text{ J}}/1460{\text{ J}}\);
\(n{\text{(N}}{{\text{H}}_{\text{4}}}{\text{Cl)}} = \frac{{5.35}}{{53.5}}/0.100{\text{ mol}}\);
\(\Delta H = + 14.6/14.6{\text{ (kJ mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept q = 105.35 \( \times \) 4.18 \( \times \) 3.50 / 1541 J.
Accept \(\Delta H\) = +15.4 / 15.4 (kJ\(\,\)mol–1)
Examiners report
Part (a) was answered well although some mentioned “dissolving” instead of “dissociating”.
In (b), the equation was well done.
In (b), the equation was well done as was (ii).
Inevitably, many omitted “pair” in (iii).
Part (c)(i) was generally correct. In (c)(ii) the carbonate ion was legitimately examined under AS 4.2.7; it was not well known – there were too many carbons with expanded octets and oxygens where the lone pairs had been missed. (In the HL specification, the carbonate ion‘s delocalization is considered.) In (iii), however, the shapes were well known.
If there was to be an error made in (d)(i), it was to omit “enthalpy” from the y-axis and some unaccountably put the correct chemicals on the line and then reversed the names products and reactants. The calculations in (d)(iii) inevitably depended on an ability to calculate and think logically.
The calculations in (e) inevitably depended on an ability to calculate and think logically.
When nitrogen gas and hydrogen gas are allowed to react in a closed container, the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst. Explain the effect of a catalyst on the rate of reaction.
Sketch the Maxwell–Boltzmann energy distribution curve for a reaction, labelling both axes and showing the activation energy with and without a catalyst.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted-Lowry theory.
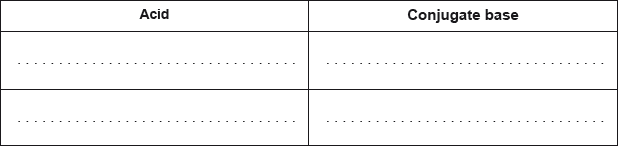
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline an experiment and its results which could be used to distinguish between a strong base and a weak base.
Markscheme
rates of forward and reverse reactions are equal / opposing changes occur at equal rates;
the concentrations of all reactants and products remain constant / macroscopic properties remain constant;
closed/isolated system;
Accept “the same” for “equal” in M1 and for “constant” in M2.
\(({K_{\text{c}}} = )\frac{{{{{\text{[N}}{{\text{H}}_3}{\text{(g)]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{(g)]}} \times {{{\text{[}}{{\text{H}}_2}{\text{(g)]}}}^3}}}\);
Ignore state symbols.
Concentration must be represented by square brackets.
The volume of the container is increased:
position of equilibrium shifts to the left/reactants and fewer moles of gas on the right hand side/pressure decreases / OWTTE;
Ammonia is removed from the equilibrium mixture:
position of equilibrium shifts to the right/products and [NH3] decreases so [N2] and [H2] must also decrease to keep Kc constant
OR
position of equilibrium shifts to the right/products and rate of reverse reaction decreases / OWTTE;
Award [1 max] if both predicted changes are correct.
Do not accept “to increase [NH3]” or reference to LCP without explanation.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Accept “energy difference between reactants and transition state”.
rate increases;
more effective/successful collisions per unit time / greater proportion of collisions effective;
alternative pathway and a lower activation energy
OR
lowers activation energy so that more particles have enough energy to react;
Do not accept just “lowers/reduces the activation energy”.
Accept “provides a surface for reacting/reactants/reaction”.
Curve showing:
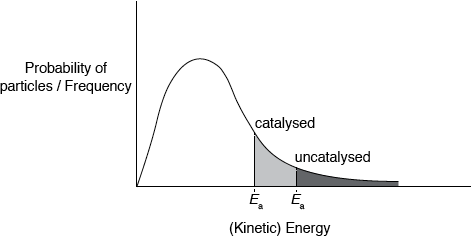
general shape of Maxwell-Boltzmann energy distribution curve and labelled y-axis: probability of particles / frequency and labelled x-axis: (kinetic)energy;
Curve must begin at zero and must not cut the x-axis on the RHS.
Accept number/fraction/proportion of particles for y-axis label, but do not accept amount or just particles.
correct position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
Shading shown in the diagram is not required for the marks.
(i) slower rate / OWTTE;
uneconomic / OWTTE;
(ii) high cost for building/maintaining plant / high energy cost of compressor /OWTTE;
Do not accept “high pressure is expensive” without justification.
Accept high pressure requires high energy.
electron pair donor;
Accept lone pair donor.
proton acceptor and partially/slightly ionized;
Accept “proton acceptor and partially/slightly dissociated”.
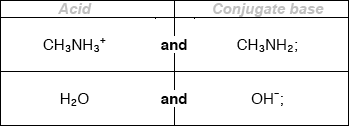
Award [1 max] for two correct acids OR two correct conjugate bases.
solutions of equal concentration;
pH measurement/UIP;
strong base has higher pH;
OR
solutions of equal concentration;
electrical conductivity measurement;
strong base has higher electrical conductivity;
OR
solutions of equal concentration;
temperature difference in neutralization reaction with a strong acid;
strong base has a greater temperature difference;
Accept reverse arguments for observations.
Examiners report
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
Across period 3, elements increase in atomic number, decrease in atomic radius and increase in electronegativity.
Define the term electronegativity.
Explain why the atomic radius of elements decreases across the period.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Suggest the pH of the solutions formed in part (c) (i).
Describe three tests that can be carried out in the laboratory, and the expected results, to distinguish between \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) and \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\).
Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
Describe the bonding and structure of sodium chloride.
State the formula of the compounds formed between the elements below.
Sodium and sulfur:
Magnesium and phosphorus:
Covalent bonds form when phosphorus reacts with chlorine to form \({\text{PC}}{{\text{l}}_{\text{3}}}\). Deduce the Lewis (electron dot) structure, the shape and bond angle in \({\text{PC}}{{\text{l}}_{\text{3}}}\) and explain why the molecule is polar.
Lewis (electron dot) structure:
Name of shape:
Bond angle:
Explanation of polarity of molecule:
Markscheme
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons;
Do not accept “element” instead of “atom/nucleus”.
Do not accept “electrons” alone.
increasing nuclear charge/increasing number of protons / increased attraction of (valence) electrons to nucleus;
electrons added are in same (outer) energy level;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
Accept \(N{a_2}O(s) + {H_2}O(l) \to 2N{a^ + }(aq) + 2O{H^ - }(aq)\).
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_3}{\text{(aq)}}\);
Accept \({P_2}{O_5}(s) + 3{H_2}O(l) \to 2{H_3}P{O_4}(aq)\).
Accept \({P_4}{O_{10}}(s) + 6{H_2}O(l) \to 4{H^ + }(aq) + 4{H_2}PO_4^ - (aq)\).
Ignore state symbols.
NaOH: > 7;
Accept any pH greater than 7.
H3PO4: < 7;
Accept any pH less than 7.
Award [1 max] if stated that “NaOH alkali/basic and H3PO4 acidic”, but pH values not given.
measuring electrical conductivity and strong acids have greater electrical
conductivity/weak acids have lower electrical conductivity;
Do not accept conductivity for electrical conductivity.
Accept explanation in terms of lightbulb in circuit.
measure pH/use universal indicator and pH higher for weak acid/pH lower for strong acid;
conduct titration with a strong base and equivalence point higher for weak acid / buffer region for weak acid;
adding a reactive metal/carbonate/hydrogen carbonate and stronger effervescence/faster reaction with strong acids;
Accept converse argument.
Accept correct example.
adding a strong base and strong acid would increase more in temperature/weak acids increase less in temperature;
Accept correct example.
Award [1 max] for three suitable tests without correct results.
Accept specific examples with given strong acid and weak acid.
Accept “addition of AgNO3 (aq) and white precipitate with HCl (aq)”.
Do not accept “smell”.
Lewis acid (only);
electron pair acceptor / not a proton donor;
Bonding: (electrostatic) attraction between oppositely charged ions;
Do not accept ionic bonding without some description.
Structure: lattice/giant structure of ions / each \({\text{N}}{{\text{a}}^ + }\) surrounded by \({\text{6 C}}{{\text{l}}^ - }\) (and vice-versa);
\({\text{N}}{{\text{a}}_2}{\text{S}}\);
\({\text{M}}{{\text{g}}_3}{{\text{P}}_2}\);
Lewis structure:
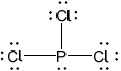 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
Do not award the mark if lone pairs are missing.
Name of shape:
(trigonal/triangular) pyramidal;
Bond angle:
\( < 109.5^\circ \);
Accept any value within the range 100°−109°.
Literature value is 100°.
Explanation of polarity:
dipoles do not cancel (as molecule is not symmetrical) / there is a net dipole (as molecule is not symmetrical) / unsymmetrical distribution of charge;
Accept suitable labelled diagram.
No ECF if original structure is incorrect.
Examiners report
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.